0 Comments
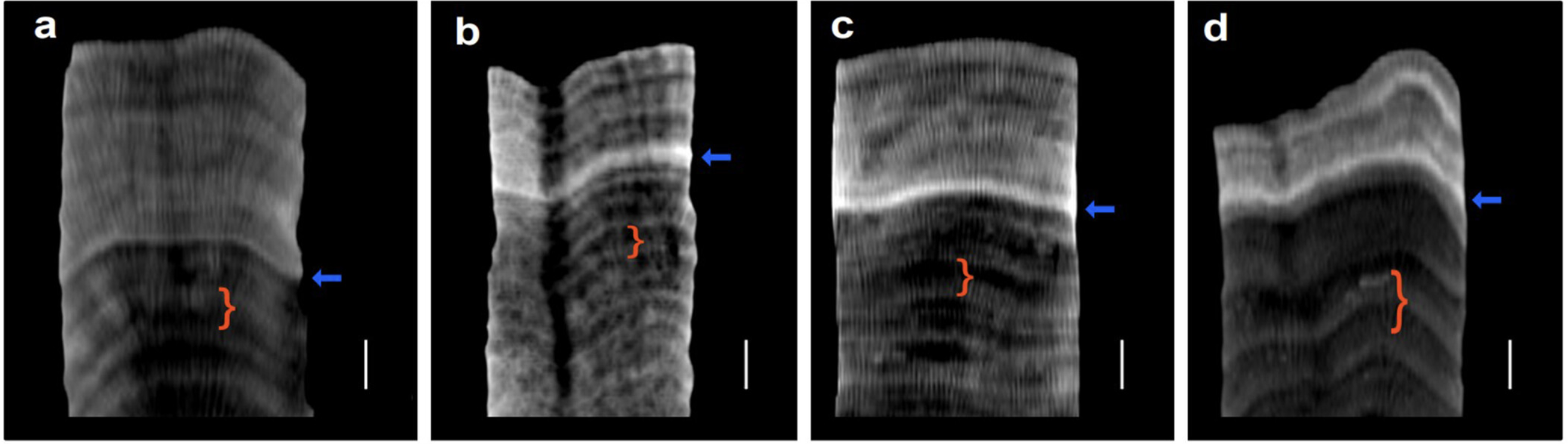
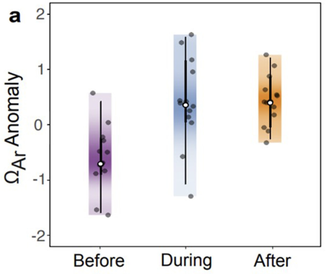 The key results: higher saturation state during and after bleaching, relative to before. The key results: higher saturation state during and after bleaching, relative to before. The first publication of work conducted entirely in the Sclero Lab is out today in L&O Letters. Sclero-lab alumnus Hanna Mantanona used Raman spectrometry to analyze coral skeletal cores with prominent bleaching-induced stress bands. Using the Raman data, Hanna calculated calcifying fluid saturation states before, during, and after the bleaching events. And the results were, well, surprising! Saturation state increased during the bleaching, and remained elevated afterwards. This paper will be a key contribution to the literature not because it provides all the answers (we don't fully know the mechanism behind this observation), but because it refutes existing notions in the literature and raises new questions. Way to go, Hanna! With funding from NSF, we will be working on a 5-year project focused on advancing analysis of coral skeletal cores. This will include the creation of a virtual core repository, developing new tools for analyzing CT scans, and engaging the public in the analyses. Read more about the project here.
 by Hannah Whitaker The physical and chemical properties of a coral’s skeleton can tell us a lot about past ocean conditions; some coral species maintain environmental records that go back hundreds of years. The Sclerochronology lab has a collection of coral cores from all over the world, and this past summer, we expanded our library to include 10 new specimens from here on ‘Oahu!  Under a permit from the Division of Aquatic Resources, our team of divers (Dr. Tom DeCarlo and graduate students Hannah Whitaker and Jess Hankins) identified and cored colonies of Porites lobata in Waimanalo Bay over the course of five dives. Lobe coral is one of three dominant coral species on the island, and its massive growth form and long-lived nature make it an excellent candidate for sclerochronology.  The drill we use is pneumatic—powered by pressurized air—and produces cores that are 5 cm in diameter, about as big around as a water bottle. Once the core is removed, we plug the hole with cement and marine epoxy to keep bioeroders out and so that the surrounding coral has a new surface over which to grow back. Within a few months, it’s like we were never there.  Back on land, we rinse, soak, sonicate (using a high-frequency sound bath to remove debris), oven-dry, and carefully wrap each core so they can be stored for later analysis. Our longest core from Waimanalo likely dates back over a hundred years, and the lab is excited to see what history is written in these growth bands.  - by Jordyn Cotton, Sclerochronology lab Master's student I recently returned from a trip to the Land Down Under where I had the opportunity to get my hands dirty with field work out on the reef. Funded by the National Science Foundation, Interdisciplinary Research Experience for Students (IRES) was a three-week program to provide graduate students with field experience and expand their knowledge of coral reef science. The first week was spent in the Atherton Tablelands at the Center for Rainforest Studies about an hour southeast of Cairns, Queensland. While living in the rainforest, we learned about the connection between the land and sea and how terrestrial activities can impact the coral communities offshore. We were introduced to some of our first Australian natives: from spiders bigger than your fist to venomous snakes and massive bats. Of course, we also had to try heaps of Aussie delicacies including Vegemite, Tim Tams biscuits, and Shapes crackers. Our first guest lecturer, Michele Barnes, from the ARC Center of Excellence for Coral Reef Studies at James Cook University, gave lectures about bridging the gap between social and ecological sciences. Workshops were then held to demonstrate how creating these ties between users of a particular ecosystem are connected to one another and how these need to be addressed to implement effective management strategies. After learning about the Australian wet tropics and meeting a ton of incredible people along the way, it was time to head offshore.  As if we weren’t already itching to get out into the water, the small nine-seat plane ride over the reef had everyone awe-struck. The sheer number of reefs and magnitude of each patch was enough to make you speechless. I cannot even fathom what flying over the nearly 1500 mile-long reef would look like. About an hour after leaving Cairns, we landed on Lizard Island, a National Park found ~16.5 miles off the Queensland coast. Home to a high-end luxury resort and a Research Station run by the Australia Museum, Lizard Island is fully protected and boasts over 2500 species of marine life including corals, fish, turtles, sharks, rays, and more. Our time at the station was filled with as much snorkeling as possible, despite the high winds typical of this time of year. Our first snorkel of the trip was in an area called clam gardens near the resort on the western side of the island. Appropriately named, large clams could be seen sprinkled amongst the vast field of corals in every shape, color, and size. Brightly colored fish flitted in and out of their hiding spots as we swam by. Photos do not do these reefs justice, but I included some anyway. We also frequently visited an area called Mermaid Cove, located at the far northern tip of the island. After being decimated by a series of bleaching events, cyclones, and a ruthless case of Crown-of-Thorns starfish, this area was now recovering and has become a haven for pioneer coral colonies. Plates of Acropora large and small were interlaced with one another and created an endless scene of new growth over piles of dead coral rubble. The high winds caused large waves and prevented us from exploring the island’s massive reef flat. One very popular daily ritual on Lizard Island is to all meet on the beach for sunset beers. While beer isn’t mandatory, this was a great way to meet and chat with other researchers from around the world and learn about their projects.  Later that first week, we were joined by Morgan Pratchett, an ecologist from James Cook University focused on coral reef disturbance. Morgan also taught us some tips for identifying corals in situ and discussed how advancements in molecular technology led to a complete overhaul of the traditional taxonomy, making some classifications obsolete. With our new tips and tricks in hand, we made our way out to Mermaid Cove to practice our coral identification by swimming along transects and pointing out corals to the genus level. While on Lizard Island, we were also tasked with our own independent projects. Coring was off the table due to a lack of permits and the weather prevented us from deploying instruments too far offshore, so we went with plan C which was to obtain water samples and test for changes in pH and alkalinity throughout a 24-hour period. With previously collected data from the reef flat at our disposal, I chose to deploy the autosampler and SeapHOx near a patch of mangroves to see how the biogeochemistry differs from a reef environment. As is the nature of field work, this first deployment wasn’t the roaring success I had hoped. The extremely low tides meant that the instruments weren’t submerged enough to collect a sample, so instead of 12 samples, I only had eight.  While we worked out the solution to this problem, our second week on Lizard Island rolled around. Individual projects were in full swing and I spent several hours in the lab working on titrating the few samples I had for alkalinity and pH data. I was also able to assist others on their projects which gave me even more hands-on experience, particularly in coral reef fish social behaviors. After consulting with the tide charts several times, we reprogrammed the autosampler to avoid sampling during those low tide hours and spaced-out sampling before redeploying the instruments at the base of the mangroves. 48 hours later, I collected the device and quickly ran all the titrations necessary to present in front of the group plus the directors of the station Lyle and Anne. Our final guest lecturer of the trip was Brad Eyre, a biogeochemist from Southern Cross University. He lectured on the importance of biogeochemistry and his work on the dissolution of carbonate sands, the foundation of coral reef ecosystems. We then ventured out for a field exercise using benthic chambers to explore how ocean acidification can impact the biogeochemical properties of the sediment near the mangrove patch.  While it may sound like this trip was all work and no play, we had plenty of time to explore. These extreme low tides afforded us with the opportunity to walk out along the beach and see exposed corals, sea cucumbers, and sea stars. I even caught a glimpse of some gorgeous Bluespotted Fantail rays and a few Epaulette sharks in just a few inch deep water. We also made the trek up to the ever historically important Cook’s Look. This daunting and steep 2.5 mile climb follows roughly the same track taken by the famous explorer Captain James Cook when he tried looking for a way to navigate out of the coral “labyrinth” that surrounded the island. One of our final days in the Great Barrier Reef was reserved for a good-old-fashion frolic amongst the corals. We headed out to Watson’s Bay and enjoyed several hours to freely swim and admire all the life below the surface. The HPU Sclero lab, in collaboration with the Center for Marine Debris Research, was awarded $319,965 from the National Science Foundation to acquire a state-of-the-art Raman spectrometer. We will use the instrument for quantifying the chemistry of coral calcification, identifying the types of plastics in water and sand samples, and in teaching labs. The instrument will also be available for external users, just get in touch if you are interested in using our Raman for your research project. Among a range of features, our instrument will have multiple lasers of various wavelengths, automated 2-d and 3-d mapping, topography mapping, and the ability to process large solids and liquids.
New paper on the interactive effect of nutrients and heat stress in sparking coral bleaching8/25/2020 I have drilled skeletal cores from massive coral colonies all around the world, from the Caribbean, to remote Pacific Islands, Taiwan, Australia, and Palau. Finding live corals was never very hard, there were always plenty to choose from and I would search for the largest (oldest) corals. Then, in 2019, I went to the Farasan Banks, in the southern Red Sea. I could certainly see the amazing diversity and coral cover that used to exist here. But, it's almost all dead, following a terrible bleaching event in 2015. The Red Sea is not hit by large storms or waves, so the corals are all standing dead, creating an eerie reef graveyard. I've never spent so much time just snorkeling over reefs to find live corals to drill. At some reefs, there were none. For the first time, I resorted to drilling cores from dead corals. And the live corals that I did find often had partial mortality scars.
So what happened? Why was this reef so devastated? Well, our new paper in Science Advances shows that an unusual conflux of heat and nutrients led to an exacerbated bleaching response. Initial strong monsoons in early 2015 caused strong upwelling, supplying the reef with excess nutrients. Then, in late summer, the monsoon winds suddenly died off earlier than usual, leading to anomalous heating. While a few extra nutrients might normally be good for corals, our paper shows that it is bad when combined with heat stress. The reason? I suspect that the corals' symbionts quickly soak up those nutrients and grow in size / reproduce. Then, when the heat stress comes, causing the symbiont photosystem to produce toxic oxygen species, the large symbiont populations are harmful to the coral, leading to exacerbated bleaching.
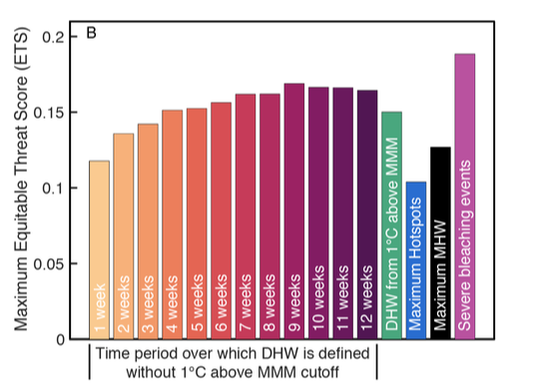
Imagine it's Sunday. You're planning a BBQ for a week from today, but the weather person on TV just forecast rain for next Sunday. How much confidence do you really have in that prediction? Turns out, that's about the confidence we can place in predicting coral bleaching from SST alone. Our new paper in PeerJ dives into these statistics and proposes some initial steps forward. The main conclusion is that we should critically evaluate our understanding of the causes of bleaching events, think beyond SST, and work together to improve our predictive ability.
|
Archives
October 2023
Categories |